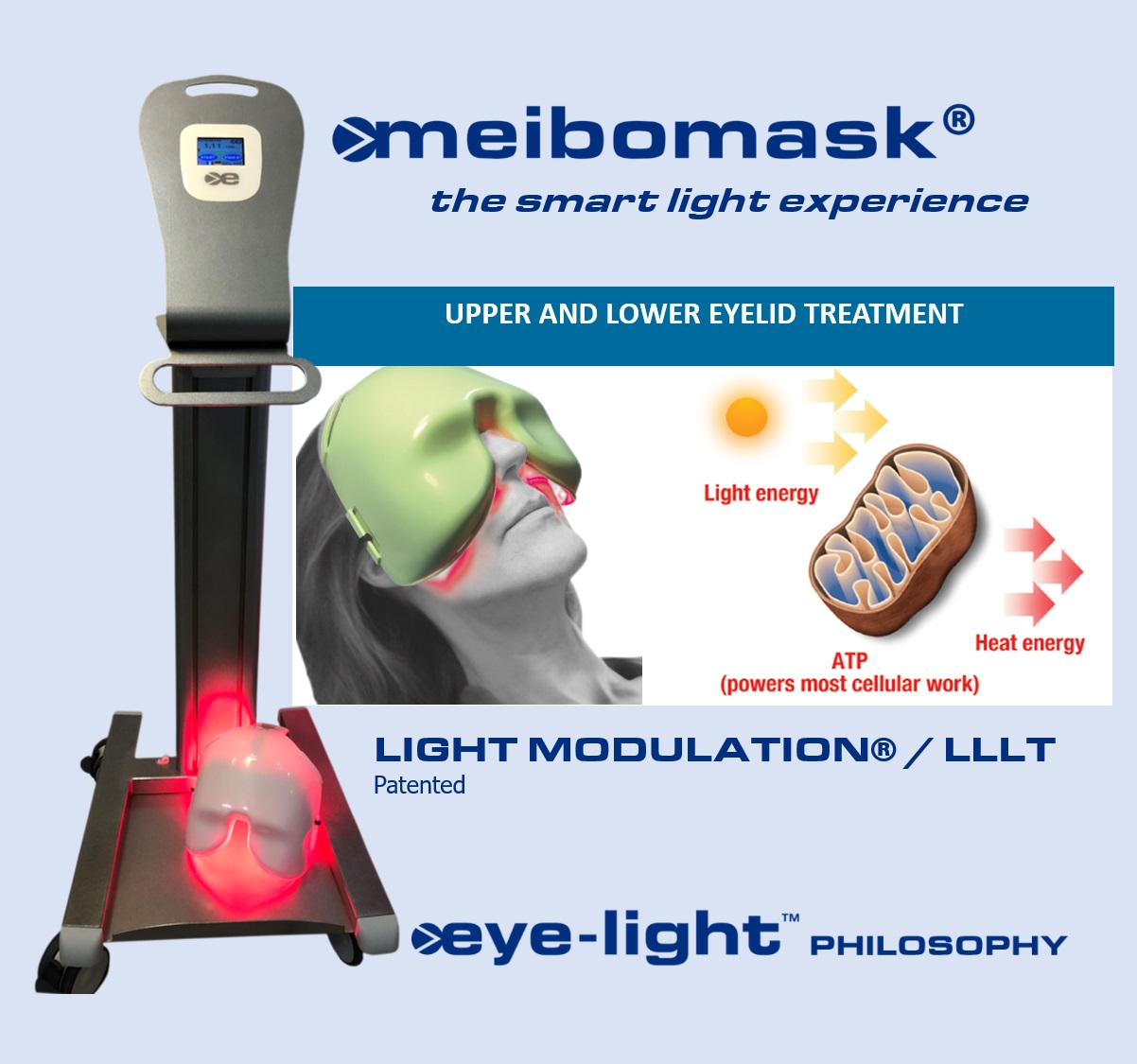
THE SMART LIGHT EXPERIENCE
![]() Click here to view the Smart Light Experience pdf
Click here to view the Smart Light Experience pdf
![]() Click here to view the Espansione Group Brochure
Click here to view the Espansione Group Brochure
![]() Click here to view the "Complete Strategy for Dry Eye Treatment" pdf
Click here to view the "Complete Strategy for Dry Eye Treatment" pdf
![]() Click here to view the My Mask protocols
Click here to view the My Mask protocols
![]() Click here to view the Be ready with Eye-Light Line 2021 Brochure
Click here to view the Be ready with Eye-Light Line 2021 Brochure
![]() Click here to view the Eye-Light Dermatology Protocols
Click here to view the Eye-Light Dermatology Protocols
ARTICLES
![]() Click here to view the ARVO Annual Meeting Abstract - June 2020 pdf
Click here to view the ARVO Annual Meeting Abstract - June 2020 pdf
![]() Click here to view the Dove Press article on IPL/LLLT treatments
Click here to view the Dove Press article on IPL/LLLT treatments
![]() Click here to view an article on Skin temperature measurement after IPL and LLLT application
Click here to view an article on Skin temperature measurement after IPL and LLLT application
![]() Click here to view the Euro Times article on the Prevalence of Meibomian Gland Dysfunction
Click here to view the Euro Times article on the Prevalence of Meibomian Gland Dysfunction
![]() Click here to view the Review of Optometry article: Shed Some Light on DED
Click here to view the Review of Optometry article: Shed Some Light on DED
![]() Click here to view the Intense Pulsed Light vs. Low-level Light Therapy
Click here to view the Intense Pulsed Light vs. Low-level Light Therapy
Click here to view videos about the Meibomask
Watch the FOX26 video
A full LLLT stand-alone machine that setup the treatment according to the MGD level. Something unique in the market.
It's more and more evident that IPL technology is going to its end. LLLT will be the future. Look at the latest literature. IPL treats only 50% of the pathology. Don’t hesitate to make a direct question to your clients … “are you happy to treat 50% of the problem ?” More and more papers say that LLLT is the most efficient treatment for MGD.
At this point a lot of attention is focused on us, because we are the ONLY company in the world that is producing and promoting THREE succesful LLLT devices and TWO systems for screening and diagnosis of Dry Eye. Each market / Each Field has its dedicated device!
Meibomask® is intended for being used in professional environments.
This patented photobiomodulation technology solicits cell’s mitochondria for a better energy production and triggers biochemical and biophysical reactions that stimulate cells to make a better protein synthesis. Thanks to the light emission of LED matrix, the tear lipid layer is increased and stabilized.
Meibomask® provides customized treatments based on the MGD severity level detected with Me-check® screening instrument. According to severity of meibomian glands loss, Meibomask® releases the correct amount of energy and treatment duration for the specific patient.
- 2000: Espansione started to design and produce IPL machines for aestetic medicine, and the LLLT technology has been patented for dermatology applications.
- 2016: the Eye-Light machine has been launched.
- 2016: The My-Mask stand alone device has been realized for self-treatment of MGD pathology.
- 2018: the Me-Check screening device has been realized for easy and fast MGD screening, plus OSDI-6 questionnaire included in the software.
- 2018: Me-Check has been awarded by Horizon2020 European Program, as the most innovative medical device.
- 2019: Eye-Light machine has been improved, by adding some new treatments to the built-in software.
- 2020: Me-Check 3D comes from the ordinary Me-Check; thanks to the cooperation with the BioEngineering Department of the University of Bologna, a new Artificial intelligence algorithm has been developed to automatically detect the MGD severity level of the patient, and some more features has been added to the software.
Until 2020: With the time passing, a lot of KOLs cooperate with us; prof. Alìo from Spain, prof. Cochener from France, Dr. Shetty from India, prof. Stonecipher from USA and prof. Buratto from Italy are some of the very important names cooperating with us.
Certifications are obtained for almost all the countries, such as Canada, Brasil, Korea, China and also FDA for USA.
In the Covid-19 period we performed 5 webinars covering Canada, USA, Europe and Asia. Very important ophthalmologists have shown their successful experience with Eye-Light , reporting excellent feedbacks on treated cases. Several hundreds of ophthalmologists attended to the webinars and a lot of orders have been collected in this period. When Covid-19 locked several countries, we got sales in Algeria, India, Argentina and Bulgaria.
Some more papers have been published about the combination with IPL and LLLT technology.
- 2020: the Meibomask has been released. A full LLLT stand-alone machine that setup the treatment according to the MGD level. Something unique in the market.
Meibomask can be used by ophthalmologists, optometrists and opticians.
It's more and more evident that IPL technology is going to its end. LLLT will be the future. Look at the latest literature.
A review study of the latest 20 papers published in the period 2015-2019 is included in the Cochrane Systematic Review, 2020!.....They say that IPL is not safe and is not efficient!!! You MUST read it carefully.
More and more papers say that LLLT is the most efficient treatment for MGD [Heiko Pult - ARVO Annual Meeting Abstract, June 2020 - or "Effects of Low-level Light Therapy at 740 nm on Dry Eye Disease In Vivo" - Hyeyoon Goo, Hoon Kim, Jin-Chul Ahn, Kyong Jin Cho - Med Laser 2019;8(2):50-58]...
At this point a lot of attention is focused on us, because we are the ONLY company in the world that is producing and promoting THREE succesful LLLT devices.

ME-CHECK 3D
![]() Click here to view the Me-Check 3D brochure
Click here to view the Me-Check 3D brochure
ARTIFICIAL INTELLIGENCE USE FOR THE PERFECT MGD EVALUATION
Please find here some informations on the NEW device Me-Check 3D.
Since 2016 Espansione Marketing is manufacturing a complete line of devices dedicated to Dry Eye screening and treatment, developed with the scientific support of internationally very well-known scientists and ophthalmologists; in 2018 the European Commission of HORIZON 2020 has chosen ME-CHECK® as the unique and most innovative device for the Dry Eye SCREENING, because it can EARLY detect and recognize the pathology and thus it can be very interesting for the ophthalmologists’, optometrists’ and opticians’ market.
Standard Me-Check is a Class I screening device.
Now we have the NEW Me-Check 3D!
--> Me-Check 3D has been developed in cooperation with the BioEngineering Department of the University of Bologna and it uses an Artificial Intelligence Algorithm for the automatic evaluation of the MGD severity level.
--> Me-Check 3D is now a class II diagnostic device, and it has been already registered in Europe, Canada, USA, Australia and other countries.....
--> Imaging acquisition has been improved; brigthness and contrast can be adjusted in a very fine way, and the AI software can recognize if the acquired picture is not good enough to be processed by the software.
--> A new centration system has been added for the best acquisition. With this accuracy is very easy to get a reliable result to perform the most accurate diagnosis.
--> The MGD severity level has a better classification and each level of the Dr Pult scale has now 3 sublevels for the most accurate MGD classification.
--> 3D view of Mebomian Glands picture available.
--> Pdf patients reports can be saved into a Google Drive platform.
--> NEW: Network Sharing Capabilities to be integrated with the most common PMS and EMR softwares.
--> DemoDex viewing app is now integrated into the software. SEE attached sample pictures.
You can find also Me-Check 3D features in the video here
ESPANSIONE GROUP INFORMATION:
Technologies:
https://www.espansionegroup.it/technologies/
Our Publications:
https://www.espansionegroup.it/bibliography/
Our Protocols:
https://www.espansionegroup.it/protocols/